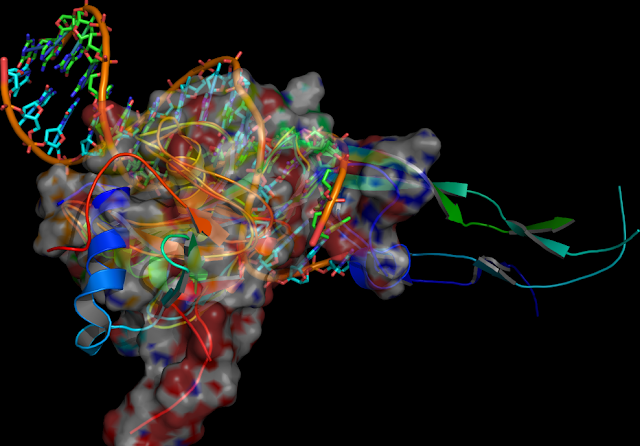
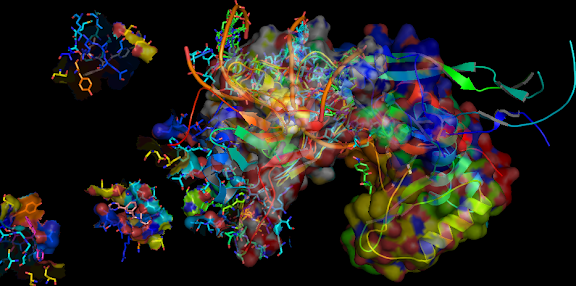
Thioredoxin: human TXN, is a oxidoreductase enzyme in the
status of a 12 kDa
cellular redox-reductase
reaction (70-kDa
in bacteria, fungi and plants), a cellular
defense mechanisms against oxidative stress of the cell, and
numerous cytosolic processes in all cells.
Txn1 is a pleiotropic
cellular causative gene factor which has numerous
functions. Chromosome 3p12-p11
shares homology with human thioredoxin gene Trx1, Trx80:
9q31.3; (§,
‡). Here the following reaction is the possible mechanisms of the thioredoxin-catalyzed reduction and re-oxidation of its characteristic cystine residues.
The TXN gene, consists of the first of 5 exons separated by 4 introns and is located 22 bp downstream from the only known basal TATA box factor TBP-2/TXNIP vitamin D(3) up-regulated protein 1-VDUP1, negatively regulating TRX function, and exhibiting cellular growth and suppressive (cancer) activity.
TRX inhibited Apoptosis signal-regulating kinase-ASK1 kinase (MAP3K5), activity, dependent on two cysteine residues in the N-terminal domain of ASK1 on the redox (regulation) forming intramolecular disulfide between the status of TXN. Two cysteine residues (N-terminal C32S or Trx C-terminal C35S and/or a Trx-CS double mutation) remaining trapped with the Ask1 as a inactive high-molecular-mass complex, blocking its reduction to release Trx from ASK1 depends on intramolecular disulfide to catalyze the reduction of the redox regulation of TRX. Trx and a thiol-specific antioxidant thioredoxin peroxidase-2 orthologue (Tpx) in various* biological phenomena is involved in redox regulation (NADPH-the thioredoxin system) of the dithiol-disulfide active site.
An apoptosis signal transduction pathway through stimulus-coupled S-nitrosation of cysteine, has two critical (almost identical) cysteine residues in the Trx redox-active center. Where a disulfide exchange reaction between oxidized Txnip [thioredoxin-interacting protein; mouse Vdup1] and reduced TXN occurs. Txnip (-when used to investigate cardiac hypertrophy) is a regulator of biomechanical signaling. Hydrogen peroxide downregulated expression is the only known function associated with an incomplete TRX response through stimulus-coupled S-nitrosation of cysteine residues. Peroxiredoxin PrxIII-'Tpx1 serves as' a tandem (dimer) thioredoxin (Trx2) and NADP-linked thioredoxin reductase (TRR2-TxnR1), are Trx mechanisms of the two electron donor system.
Cytosolic caspase-3 was maintained by S-nitrosation, consistent with cytosolic and mitochondria, Trx-1 contain equivalent Trx systems, which enabled identification of caspase-3 substrates where TXN may regulate S-nitrosation with the redox center of TXN specific (C73S) to Nitric oxide-NO cellular signal transduction associated with inhibition of apoptosis or mutant Trx neurotoxicity. EGCG° (epigallocatechin-3-gallate) may be useful in cell survival on caspase-(3_dependent)-neuronal apoptosis where a membrane reaction, a reduced hormesis consequently triggers the apoptosis effect and direct or indirectly numerous protein-protein interactions and basal cofactor substrates which occur between caspase-3 and Trx. The effect of exercise training via activation of caspase-3 has a decrease in superoxide, and increase of Trx-1 levels in brain. Protection from mechanical stress identified, NSF- N-ethylmaleimide transduced into a TRX peroxidase response via mechanical force of a typical transnitrosylated Casp3, attenuated Trx1 2-cysteines which directly transnitrosylates Peroxiredoxins. C32S ( redox potential) was identified as thiol-reducing system, which lacks reducing activitiy (non-active C69S and Cys(73) both monomeric) or a reversible regulating function in the presence of caspase 3 activity is a process found in the presence of NADP and TrxR.
There are at least two thioredoxin reductive or oxidative** (reductases / peroxiredoxin) regulated systems. The mutant 32CXXC35' motif of thioredoxin nitrosation sites, where two cysteines are separated by two other amino acids, and codes for an additional three cysteines where the Cys 62/C73S (not monomers) sidechain the active site of Cys 62 also can form several disulphides and be modified by the carbon-bonded sulfhydryl, where the thiol reducing system, was evident.
Intracellular TRX/ADF (Adult T cell leukemia-derived factor HTLV-I) can regulate cell nuclei, protein-nucleic acid interactions. Transnitrosylation and denitrosylation is a reversible Post-translational (PTM) altered by redox modification of different cysteine residues (C32-73S) in Trx1, S-nitrosation or its interactions with other proteins and DNA-dependent nuclear processes. NFKappaB - REF-1 redox factor 1 involving Cys62, in the two complexes, are correlated as N ⇔ C-terminal responses with TRX-1 nuclear migration through the reduction of a pleiotropic cellular factor. TRX redox activities of protein-protein cysteine residues is identical to a DNA repair enzyme through various cytoplasmic aspects mediating cellular responses in the 'nucleus'. The DNA binding activity and transactivation of 'AP-1' activator proteins (JUN-proto* oncogen) depends on the reduction between the sulfhydryl of cysteines to keep Trx1 reduced, is demonstrated in cells. Selenium-dependent seleneocysteine based peroxidase reductants, reduce Lipoic acid stereoselectively under the same TRX rather than GSH-PX1-glutathione peroxidase oxidative stress conditions. Sense-antisense (TRX) antiapoptoitic interactions nitrosylated at Cys73 are attenuated and integrated into the host cell under oxidative conditions, in which thioredoxin (TRX), and a cellular TRX reducing catalyst agent (DTT-redox reagent) to S-nitrosoglutathione (GSNO) intermediate via cysteine residues 'influences'-catalyst mediated (post-translational modifications) PTMs; and possibly 1,25D(3)-Calcitriol; NADPH:oxygen oxidoreductases correlated with (Trx-1) a protein disulfide oxidoreductase.
Peroxynitrite** converts superoxide to hydrogen peroxide (H2O2)-induced Trx degradation, in concentrations that detoxify reactive oxygen species (ROS), demonstrated by superoxide dismutases (SOD)-catalase: ↩ and peroxidases, converting superoxide to hydrogen peroxide which is decomposed to water plus oxidized thioredoxin to maintain the anti-apoptotic (C62) function of thioredoxins additional five sulfhydryl group thiols in the fully reduced state, in a Trx-dependent manner. Reactive oxygen species (ROS) can cause DNA damage, and uncontrolled cellular proliferation or apoptotic death of cancer cells.The NADPH (Trx system) oxidizing substrate-dependent reduction of Thioredoxin reductase-TrxR has a reversibly modulated role in restoration of GR (glucocorticoid receptor) function, and DNA binding domain.
(Click on image to Zoom)
 Secreted Trx may participate in removing inhibitors of
collagen-degrading metalloproteinases. PMID: 14503974 the molecular
mechanisms underlying functional the TR1-Trx1 redox pair and
structure determination of an active site of the ligand
mini-stromelysin-1 TR-1 augmentation composed of TR (Trx
reductase activities) the main function of TR1 here is to
reduce Trx1 also validated as a ligand PMID; 23105116, have
been characterized between ligand bound and free structures
PMID; 20661909, for specific isolation of C35S
selenocysteine (SeCys)-containing protein shows
the best docking position found, consists of one strand at
position [PROline]76:A.side chain: from the four-stranded
antiparallel beta sheet was with wild-type TrxA C32-35S
located in the Thioredoxin_fold (PDB accession code 1XOB:
PMID: 15987909) , TR1 as a single hybrid PDB (Cys32
and Cys35 for Trx1, and for TR1) pubmed/20536427 investigate
the possible mechanism. {{{During this reduction, the
thiol-disulfide oxidoreductase thioredoxin-1 (Trx1) linked
Secreted Trx may participate in removing inhibitors of
collagen-degrading metalloproteinases. PMID: 14503974 the molecular
mechanisms underlying functional the TR1-Trx1 redox pair and
structure determination of an active site of the ligand
mini-stromelysin-1 TR-1 augmentation composed of TR (Trx
reductase activities) the main function of TR1 here is to
reduce Trx1 also validated as a ligand PMID; 23105116, have
been characterized between ligand bound and free structures
PMID; 20661909, for specific isolation of C35S
selenocysteine (SeCys)-containing protein shows
the best docking position found, consists of one strand at
position [PROline]76:A.side chain: from the four-stranded
antiparallel beta sheet was with wild-type TrxA C32-35S
located in the Thioredoxin_fold (PDB accession code 1XOB:
PMID: 15987909) , TR1 as a single hybrid PDB (Cys32
and Cys35 for Trx1, and for TR1) pubmed/20536427 investigate
the possible mechanism. {{{During this reduction, the
thiol-disulfide oxidoreductase thioredoxin-1 (Trx1) linked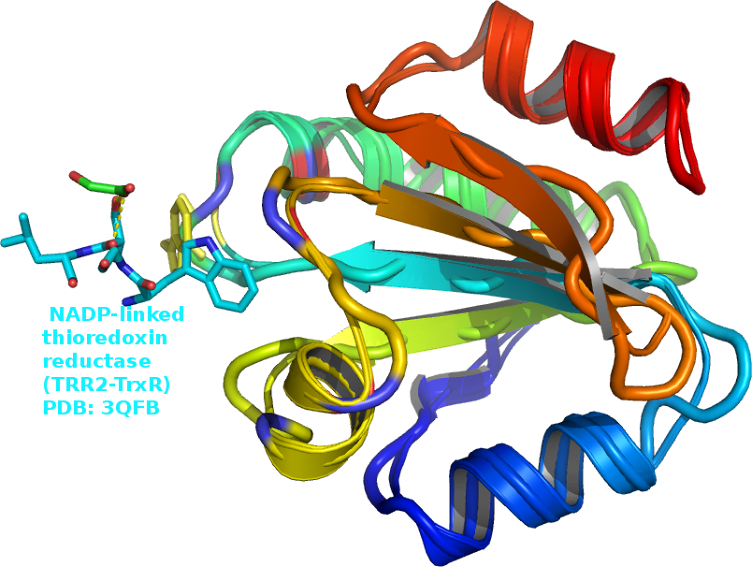 thioredoxin reductase (TRR2) a working model suggesting that
deregulation of the thioredoxin reductase TXNRD1 and|}}} its
characteristic substrate thioredoxin (TR [1]), concomitant
with diminution of their Trx reductase cellular contents is
highly related to glutamate excitotoxicity PMID: 20620191;
TR1: hStromelysin-1
thioredoxin reductase (TRR2) a working model suggesting that
deregulation of the thioredoxin reductase TXNRD1 and|}}} its
characteristic substrate thioredoxin (TR [1]), concomitant
with diminution of their Trx reductase cellular contents is
highly related to glutamate excitotoxicity PMID: 20620191;
TR1: hStromelysin-1
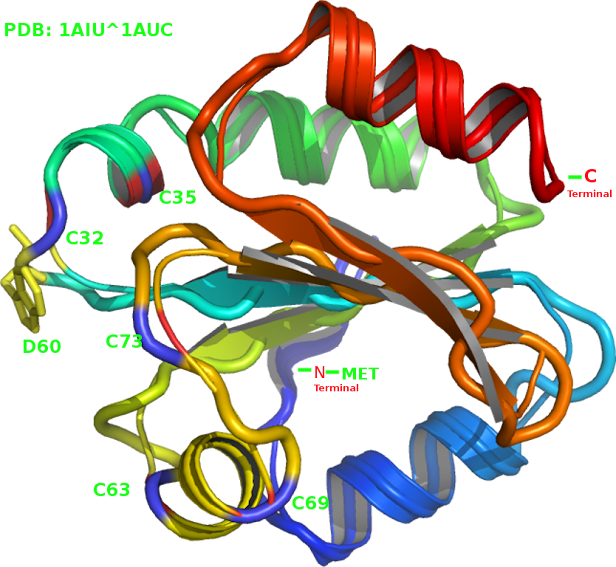 An ET
(electron transfer) mechanism from NADPH and another enzyme
thioredoxin reductase pubmed/17369362 the charged residue
aspartate D60 (Fig.2) pubmed/9369469/ plays a role in the
degradation of proteins and in apoptotic processes induced by
oxidative stress PMID: 16263712 to determine the effect
of zerumbone
ZSD1 (from shampoo ginger; Name: Alpha-humulene)
on NADP-malate dehydrogenase,
An ET
(electron transfer) mechanism from NADPH and another enzyme
thioredoxin reductase pubmed/17369362 the charged residue
aspartate D60 (Fig.2) pubmed/9369469/ plays a role in the
degradation of proteins and in apoptotic processes induced by
oxidative stress PMID: 16263712 to determine the effect
of zerumbone
ZSD1 (from shampoo ginger; Name: Alpha-humulene)
on NADP-malate dehydrogenase, TRX
dependent oxidoreductase, that NADPH does not contain.
Monomeric Thioredoxin is present across phyla from humans to
plants PMID: 20661909, 11012661 mediated in vivo by
thioredoxin-catalyzed reduction and re-oxidation of cystine
residues PubMed id: 10196131 (Fig.3-PDB: 1CIV,
NADP). Trx is able to activate vegetal NADP-malate
dehydrogenase PMID: 3170595 (excluding the initial methionine)
Met is located at the N-terminal - PMID: 11807942, 2684271. A relatively rigid local configuration for the TRX-aspartate residue D60
is found but which implies that the (NADP-TrxR) protein fluctuates among
the numerous protein models and mutations over the time scales
fluctuations.
TRX
dependent oxidoreductase, that NADPH does not contain.
Monomeric Thioredoxin is present across phyla from humans to
plants PMID: 20661909, 11012661 mediated in vivo by
thioredoxin-catalyzed reduction and re-oxidation of cystine
residues PubMed id: 10196131 (Fig.3-PDB: 1CIV,
NADP). Trx is able to activate vegetal NADP-malate
dehydrogenase PMID: 3170595 (excluding the initial methionine)
Met is located at the N-terminal - PMID: 11807942, 2684271. A relatively rigid local configuration for the TRX-aspartate residue D60
is found but which implies that the (NADP-TrxR) protein fluctuates among
the numerous protein models and mutations over the time scales
fluctuations.
Trx (thioredoxin) a redox-regulating protein also controls the antioxidant enzyme activity of the main
cellular antioxidant enzymes (AOE)
superoxide dismutase (SOD) and catalase.[↩]
(Reference: 1-189)
The TXN gene, consists of the first of 5 exons separated by 4 introns and is located 22 bp downstream from the only known basal TATA box factor TBP-2/TXNIP vitamin D(3) up-regulated protein 1-VDUP1, negatively regulating TRX function, and exhibiting cellular growth and suppressive (cancer) activity.
TRX inhibited Apoptosis signal-regulating kinase-ASK1 kinase (MAP3K5), activity, dependent on two cysteine residues in the N-terminal domain of ASK1 on the redox (regulation) forming intramolecular disulfide between the status of TXN. Two cysteine residues (N-terminal C32S or Trx C-terminal C35S and/or a Trx-CS double mutation) remaining trapped with the Ask1 as a inactive high-molecular-mass complex, blocking its reduction to release Trx from ASK1 depends on intramolecular disulfide to catalyze the reduction of the redox regulation of TRX. Trx and a thiol-specific antioxidant thioredoxin peroxidase-2 orthologue (Tpx) in various* biological phenomena is involved in redox regulation (NADPH-the thioredoxin system) of the dithiol-disulfide active site.
An apoptosis signal transduction pathway through stimulus-coupled S-nitrosation of cysteine, has two critical (almost identical) cysteine residues in the Trx redox-active center. Where a disulfide exchange reaction between oxidized Txnip [thioredoxin-interacting protein; mouse Vdup1] and reduced TXN occurs. Txnip (-when used to investigate cardiac hypertrophy) is a regulator of biomechanical signaling. Hydrogen peroxide downregulated expression is the only known function associated with an incomplete TRX response through stimulus-coupled S-nitrosation of cysteine residues. Peroxiredoxin PrxIII-'Tpx1 serves as' a tandem (dimer) thioredoxin (Trx2) and NADP-linked thioredoxin reductase (TRR2-TxnR1), are Trx mechanisms of the two electron donor system.
Cytosolic caspase-3 was maintained by S-nitrosation, consistent with cytosolic and mitochondria, Trx-1 contain equivalent Trx systems, which enabled identification of caspase-3 substrates where TXN may regulate S-nitrosation with the redox center of TXN specific (C73S) to Nitric oxide-NO cellular signal transduction associated with inhibition of apoptosis or mutant Trx neurotoxicity. EGCG° (epigallocatechin-3-gallate) may be useful in cell survival on caspase-(3_dependent)-neuronal apoptosis where a membrane reaction, a reduced hormesis consequently triggers the apoptosis effect and direct or indirectly numerous protein-protein interactions and basal cofactor substrates which occur between caspase-3 and Trx. The effect of exercise training via activation of caspase-3 has a decrease in superoxide, and increase of Trx-1 levels in brain. Protection from mechanical stress identified, NSF- N-ethylmaleimide transduced into a TRX peroxidase response via mechanical force of a typical transnitrosylated Casp3, attenuated Trx1 2-cysteines which directly transnitrosylates Peroxiredoxins. C32S ( redox potential) was identified as thiol-reducing system, which lacks reducing activitiy (non-active C69S and Cys(73) both monomeric) or a reversible regulating function in the presence of caspase 3 activity is a process found in the presence of NADP and TrxR.
There are at least two thioredoxin reductive or oxidative** (reductases / peroxiredoxin) regulated systems. The mutant 32CXXC35' motif of thioredoxin nitrosation sites, where two cysteines are separated by two other amino acids, and codes for an additional three cysteines where the Cys 62/C73S (not monomers) sidechain the active site of Cys 62 also can form several disulphides and be modified by the carbon-bonded sulfhydryl, where the thiol reducing system, was evident.
Intracellular TRX/ADF (Adult T cell leukemia-derived factor HTLV-I) can regulate cell nuclei, protein-nucleic acid interactions. Transnitrosylation and denitrosylation is a reversible Post-translational (PTM) altered by redox modification of different cysteine residues (C32-73S) in Trx1, S-nitrosation or its interactions with other proteins and DNA-dependent nuclear processes. NFKappaB - REF-1 redox factor 1 involving Cys62, in the two complexes, are correlated as N ⇔ C-terminal responses with TRX-1 nuclear migration through the reduction of a pleiotropic cellular factor. TRX redox activities of protein-protein cysteine residues is identical to a DNA repair enzyme through various cytoplasmic aspects mediating cellular responses in the 'nucleus'. The DNA binding activity and transactivation of 'AP-1' activator proteins (JUN-proto* oncogen) depends on the reduction between the sulfhydryl of cysteines to keep Trx1 reduced, is demonstrated in cells. Selenium-dependent seleneocysteine based peroxidase reductants, reduce Lipoic acid stereoselectively under the same TRX rather than GSH-PX1-glutathione peroxidase oxidative stress conditions. Sense-antisense (TRX) antiapoptoitic interactions nitrosylated at Cys73 are attenuated and integrated into the host cell under oxidative conditions, in which thioredoxin (TRX), and a cellular TRX reducing catalyst agent (DTT-redox reagent) to S-nitrosoglutathione (GSNO) intermediate via cysteine residues 'influences'-catalyst mediated (post-translational modifications) PTMs; and possibly 1,25D(3)-Calcitriol; NADPH:oxygen oxidoreductases correlated with (Trx-1) a protein disulfide oxidoreductase.
Peroxynitrite** converts superoxide to hydrogen peroxide (H2O2)-induced Trx degradation, in concentrations that detoxify reactive oxygen species (ROS), demonstrated by superoxide dismutases (SOD)-catalase: ↩ and peroxidases, converting superoxide to hydrogen peroxide which is decomposed to water plus oxidized thioredoxin to maintain the anti-apoptotic (C62) function of thioredoxins additional five sulfhydryl group thiols in the fully reduced state, in a Trx-dependent manner. Reactive oxygen species (ROS) can cause DNA damage, and uncontrolled cellular proliferation or apoptotic death of cancer cells.The NADPH (Trx system) oxidizing substrate-dependent reduction of Thioredoxin reductase-TrxR has a reversibly modulated role in restoration of GR (glucocorticoid receptor) function, and DNA binding domain.
(Click on image to Zoom)
 Secreted Trx may participate in removing inhibitors of
collagen-degrading metalloproteinases. PMID: 14503974 the molecular
mechanisms underlying functional the TR1-Trx1 redox pair and
structure determination of an active site of the ligand
mini-stromelysin-1 TR-1 augmentation composed of TR (Trx
reductase activities) the main function of TR1 here is to
reduce Trx1 also validated as a ligand PMID; 23105116, have
been characterized between ligand bound and free structures
PMID; 20661909, for specific isolation of C35S
selenocysteine (SeCys)-containing protein shows
the best docking position found, consists of one strand at
position [PROline]76:A.side chain: from the four-stranded
antiparallel beta sheet was with wild-type TrxA C32-35S
located in the Thioredoxin_fold (PDB accession code 1XOB:
PMID: 15987909) , TR1 as a single hybrid PDB (Cys32
and Cys35 for Trx1, and for TR1) pubmed/20536427 investigate
the possible mechanism. {{{During this reduction, the
thiol-disulfide oxidoreductase thioredoxin-1 (Trx1) linked
Secreted Trx may participate in removing inhibitors of
collagen-degrading metalloproteinases. PMID: 14503974 the molecular
mechanisms underlying functional the TR1-Trx1 redox pair and
structure determination of an active site of the ligand
mini-stromelysin-1 TR-1 augmentation composed of TR (Trx
reductase activities) the main function of TR1 here is to
reduce Trx1 also validated as a ligand PMID; 23105116, have
been characterized between ligand bound and free structures
PMID; 20661909, for specific isolation of C35S
selenocysteine (SeCys)-containing protein shows
the best docking position found, consists of one strand at
position [PROline]76:A.side chain: from the four-stranded
antiparallel beta sheet was with wild-type TrxA C32-35S
located in the Thioredoxin_fold (PDB accession code 1XOB:
PMID: 15987909) , TR1 as a single hybrid PDB (Cys32
and Cys35 for Trx1, and for TR1) pubmed/20536427 investigate
the possible mechanism. {{{During this reduction, the
thiol-disulfide oxidoreductase thioredoxin-1 (Trx1) linked thioredoxin reductase (TRR2) a working model suggesting that
deregulation of the thioredoxin reductase TXNRD1 and|}}} its
characteristic substrate thioredoxin (TR [1]), concomitant
with diminution of their Trx reductase cellular contents is
highly related to glutamate excitotoxicity PMID: 20620191;
TR1: hStromelysin-1
thioredoxin reductase (TRR2) a working model suggesting that
deregulation of the thioredoxin reductase TXNRD1 and|}}} its
characteristic substrate thioredoxin (TR [1]), concomitant
with diminution of their Trx reductase cellular contents is
highly related to glutamate excitotoxicity PMID: 20620191;
TR1: hStromelysin-1 An ET
(electron transfer) mechanism from NADPH and another enzyme
thioredoxin reductase pubmed/17369362 the charged residue
aspartate D60 (Fig.2) pubmed/9369469/ plays a role in the
degradation of proteins and in apoptotic processes induced by
oxidative stress PMID: 16263712 to determine the effect
of zerumbone
ZSD1 (from shampoo ginger; Name: Alpha-humulene)
on NADP-malate dehydrogenase,
An ET
(electron transfer) mechanism from NADPH and another enzyme
thioredoxin reductase pubmed/17369362 the charged residue
aspartate D60 (Fig.2) pubmed/9369469/ plays a role in the
degradation of proteins and in apoptotic processes induced by
oxidative stress PMID: 16263712 to determine the effect
of zerumbone
ZSD1 (from shampoo ginger; Name: Alpha-humulene)
on NADP-malate dehydrogenase, TRX
dependent oxidoreductase, that NADPH does not contain.
Monomeric Thioredoxin is present across phyla from humans to
plants PMID: 20661909, 11012661 mediated in vivo by
thioredoxin-catalyzed reduction and re-oxidation of cystine
residues PubMed id: 10196131 (Fig.3-PDB: 1CIV,
NADP). Trx is able to activate vegetal NADP-malate
dehydrogenase PMID: 3170595 (excluding the initial methionine)
Met is located at the N-terminal - PMID: 11807942, 2684271. A relatively rigid local configuration for the TRX-aspartate residue D60
is found but which implies that the (NADP-TrxR) protein fluctuates among
the numerous protein models and mutations over the time scales
fluctuations.
TRX
dependent oxidoreductase, that NADPH does not contain.
Monomeric Thioredoxin is present across phyla from humans to
plants PMID: 20661909, 11012661 mediated in vivo by
thioredoxin-catalyzed reduction and re-oxidation of cystine
residues PubMed id: 10196131 (Fig.3-PDB: 1CIV,
NADP). Trx is able to activate vegetal NADP-malate
dehydrogenase PMID: 3170595 (excluding the initial methionine)
Met is located at the N-terminal - PMID: 11807942, 2684271. A relatively rigid local configuration for the TRX-aspartate residue D60
is found but which implies that the (NADP-TrxR) protein fluctuates among
the numerous protein models and mutations over the time scales
fluctuations.(Reference: 1-189)