Catalase (CAT)
is converted by decomposition and intracellular localization
relationships of the main
cellular antioxidant enzyme system like superoxide
dismutase (SOD),
peroxiredoxins (Prdx), and
glutathione peroxidase (GPX) are
peroxisomal matrix enzymes in the cytoplasm, translocated
to the peroxisomes to catalyze hydrogen peroxide H2O2 which is decomposed
to oxygen and water, locus: 11p13 (§,
‡). Unlike catalase, the objective of this communication, SOD which
prevents
the formation of Hydroxyl
radicals - (HRGT)
determined from constant of O2.-
dismutation, and generation of reversibly inactive (CAT)-compound
II, Panax
ginseng could induce both transcription factors. Catalase
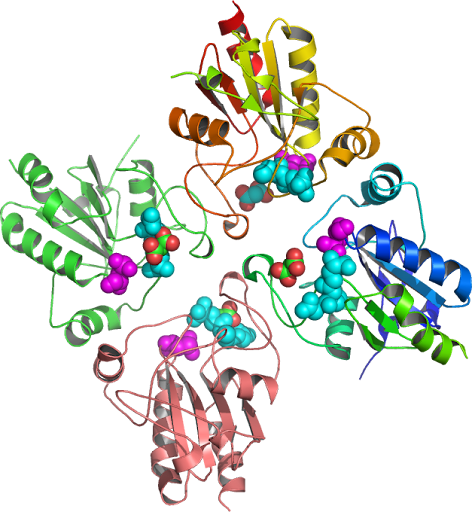
is composed of four identical subunits each of the subunits
binds one heme-containing active site, and produces two catalase compounds
HPI and HPII (PDB: 1p80)
is flipped 180
degrees » with respect to the orientation of the heme related
to the « root mean square to the structure of
catalase, (Mutation Location)
from peroxisomal catalases inactive state in compound II NADP+(H)
binding pockets inverted remains similar to the structure of the
wild type (Val111, PDB:1A4E, KatG)
orientation on the heme proximal
(PDB: 1GGK) side, inactivate
catalase can be prevented by melatonin.
Catalase (CAT; EC 1.11.1.6)
a free radical scavenging enzyme (FRSE) is a
scavenger of H2O2.
Protoporphyrin - (ZnPPIX) (PDB: 1H6N), from
a heme group of the 'heme-pathway, which forms catalase,' is a
scavenger of antioxidant
(HO-1-HMOX1)
heme oxygenase,
involving ROS.
Catalase is part of the enzymatic defense system
constituting the primary
defense against ROS, zinc
protoporphyrin IX (ZnPPIX) is
an inhibitor of (HO-1) heme oxygenase. Catalase protects
the cell from oxidative damage by
the accumulation of cellular reactive oxygen species (ROS)
generation systems, those peroxisomal
enzymes that breaks
down hydrogen peroxide after H(2)O(2) exposure, and thereby mitigates*
(some contradictory*
results) the toxic effects of hydrogen peroxide. In the process (The
typical hydroperoxidases (CAT) known as Compound I)
of the substrate of catalase, NADP+ (an inactive
state, compound
II) is replaced by another molecule of NADP(H) to provide
protection of catalase against inactivation
by (H2O2) hydrogen peroxide. Erythrocyte
[Human erythrocyte catalase (HEC), The NADPH-binding
sites were empty - PDB: 1F4J, 1QQW] and plasma indices
(enzymatic-antioxidants)
initially implies the thiobarbituric acid-reacting substances (TBARS)
based on reaction with hydroxyl radicals (OH) can
release thiobarbituric acid, TBAR
inhibition measures
malondialdehyde (MDA -
impact of coenzyme Q10)
correlated (with MPO-myeloperoxidase activity
-generating ROS) as co-variable,
by which mulberry
leaf polysaccharide (MLPII) via the decomposition of (certain)
MDA,
products of lipid
peroxidation (LPO)
were reduced. Comparisons were to specific activities of catalase (SNP) single
nucleotide polymorphisms
(CAT-C-262
(rs1001179)
the low-risk allele)
of genetic variants in both, promoter a common C/T
polymorphism (262-C/T), and in nine - exonic - regions
and its boundaries, occur frequently associated distally in genomic
mutations, similar to those of normal catalase
demonstrating changes
in catalase protein level targeted to the peroxisomal matrix. The 262-C/T CAT low-risk allele is hypothetically related to the lower risk variant allele CAT Tyr308 G to A point mutation ineducable in the Japanese acatalasemia allele. The
common C/T polymorphism can be targeted by
dietary and/or
pharmacological antioxidants, and the endogenous
antioxidant defense enzymes
concentration can prevent cellular lipid (LPO) peroxidative
reactions occurring. Catalase is a homotetramer
complex of 4 identical monofunctional
subunits. Catalase is located at the peroxisome
of human cells associated with several (PBDs)-peroxisomal
biogenesis disorders commonly caused by mutations in the PEX genes,
peroxisomal targeting signal 1 (PTS1)
protein affecting in peroxisomal biogenesis,
the monomeric to homotetrameric transition in the forms of
peroxisome biogenesis
disorder. PBDs also include Acatalasemia the only disease known to be
caused by the (CAT) gene. In human catalase, the antioxidant heme
enzyme, is localized in the cytoplasm to the peroxisome, nucleus, or linked with
mitochondria which in most cells lack catalase (Peroxisomes do not contain DNA), its mitochondrial fraction (microperoxisome), a secondary phenomena shows physiological decline, aging and age-related reactions in mitochondrial function and disfunction.
NADPH is
required for the prevention of forming an inactive
state of the enzyme. Antioxidative defence mechanisms, capacity and
redox cycle enzyme
activities increasing with Tc treatment Tinospora
cordifolia (Tc), T and B cells and antibody. Both RBCs and
plasma were measured on parameters of oxidative stress. Syzygium cumini
aqueous leaves extract (ASc) was able to remove oxidant
species in a hyperglycemic state generated in red blood cells
RBC-CAT levels. Catalase alone is unable to prevent in a
hyperglycemic state. Macrophages
recruit other types of immune cells such as lymphocytes white blood
cells (WBCs). Catalase is dependent on the family of NADPH-binding
catalases for function, the prevention and reversal of
inactivation by its toxic substrate (H2O2) hydrogen peroxide. Amyloid-beta binds
catalase and inhibits
(H2O2)
hydrogen peroxide, a reactive oxygen species, breakdown
through efficient dismutation,
and malonaldelhyde (MDA) determined in plasma, as
well as another member of the oxidoreductase family, myeloperoxidase
(MPO (EC 1.11.1.7))
converting H(2)O(2), the reducing equivalents produces (HOCl)
hypochlorous acid a mechanism of
cell-mediated antimicrobial immune defense for monofunctional
catalases one of three subgroups related to catalase deficiency in
humans, in micro-organisms manganese-containing catalases ('large
catalases') determining in part the bifunctional activity of (KatG, PDB:1X7U)
represented by bifunctional
(heme) catalase-peroxidase
based Bacterial-resistance
mechanisms. Peroxiredoxins (Prxs, EC 1.11.1.21),
bifunctional
catalase-peroxidases (KatGs) two
organelle
systems are antioxidant enzymes of the peroxiredoxin family that oxidize and reduce H(2)O(2)
hydrogen peroxide thereby modulating the catalase
reaction, KatGs are not found
in plants and animals. Trx (thioredoxin) a redox-regulating protein also controls the antioxidant enzyme activity of the main
cellular antioxidant enzymes (AOE)
superoxide dismutase (SOD) and catalase.
The function of NADPH bound
to Catalase.
 The cytosine to thymidine transition of nucleotide-262 (-262C>T)
Computer analysis indicated that the two variants bound promoter the
Ile (-262 C/T) and (B) Ile-262 in
the 5'-flanking
region carrying the T allele best captured and characterized the
generation of the hydroxyl radical site in (PDB: 1DGB), (CAT) -[GLU] 330C>T transition, is known also as -262C>T. The
'T allele in comparison to the C allele' is a common C/T
polymorphism frequency in the promoter
region association was observed between genotypes for locus11p13 risk alleles acatalasemia mutation Asp
(37C>T in exon 9) was hypothetically related to the lower risk Japanese acatalasemia allele Tyr308 a single G to A (see: rs7947841 to evaluate the link to rs769214) point mutation ineducable or near exon 9 (TC,
CC, TT) of the CAT gene to which variant changes in the promoter region
C/T-262 polymorphism are more closely related to CAT T/C at codon 389 in exon 9 (rs769217)
polymorphism did not differ significantly from
those of healthy controls in both promoter (-262 C/T) and
in exonic (ASP-389 C/T)
regions of the catalase (CAT).
The cytosine to thymidine transition of nucleotide-262 (-262C>T)
Computer analysis indicated that the two variants bound promoter the
Ile (-262 C/T) and (B) Ile-262 in
the 5'-flanking
region carrying the T allele best captured and characterized the
generation of the hydroxyl radical site in (PDB: 1DGB), (CAT) -[GLU] 330C>T transition, is known also as -262C>T. The
'T allele in comparison to the C allele' is a common C/T
polymorphism frequency in the promoter
region association was observed between genotypes for locus11p13 risk alleles acatalasemia mutation Asp
(37C>T in exon 9) was hypothetically related to the lower risk Japanese acatalasemia allele Tyr308 a single G to A (see: rs7947841 to evaluate the link to rs769214) point mutation ineducable or near exon 9 (TC,
CC, TT) of the CAT gene to which variant changes in the promoter region
C/T-262 polymorphism are more closely related to CAT T/C at codon 389 in exon 9 (rs769217)
polymorphism did not differ significantly from
those of healthy controls in both promoter (-262 C/T) and
in exonic (ASP-389 C/T)
regions of the catalase (CAT).  Tyr 370
resolves the 25 A-long (hydrogen peroxide) channel a constriction or
narrowing of the channel leading to the heme cavity ('Parameters)
situated in the entrance channel to a heme protoporphyrin (ZnPPIX)
(PDB: 1H6N) from a heme group, capable of heme biosynthesis'
in a wide range of organisms convert it into into heme b,
protoporphyrin IX-heme.
Two channels lead close to the distal side. A third channel
reaching the heme proximal side
Tyr 370, Ile-262
is proposed as a the 'PDB: 1DGB - variant with a substituted residue in the ASP 178 to the (Met)
D181E variant PDB 1p80'.
These differences include the structure of the variant protein Val111Ala
(Saccharomyces cerevisiae) related supports the existence of the 'Heme and NADP(H) binding
pockets'. The omission of a 20-residue PDB: 1F4J, (1QQW) segment
corresponds to the N-terminal (blue) of catalase from human
erythrocytes (HEC), or in a C-terminal
(red) domain organized with an extra flavodoxin-like
fold
topology may provide with weak coordination the N- or
C-terminal, that allows scrutiny of the origins (topology) in
this report of what would otherwise remain speculative or determined with further verification.
Tyr 370
resolves the 25 A-long (hydrogen peroxide) channel a constriction or
narrowing of the channel leading to the heme cavity ('Parameters)
situated in the entrance channel to a heme protoporphyrin (ZnPPIX)
(PDB: 1H6N) from a heme group, capable of heme biosynthesis'
in a wide range of organisms convert it into into heme b,
protoporphyrin IX-heme.
Two channels lead close to the distal side. A third channel
reaching the heme proximal side
Tyr 370, Ile-262
is proposed as a the 'PDB: 1DGB - variant with a substituted residue in the ASP 178 to the (Met)
D181E variant PDB 1p80'.
These differences include the structure of the variant protein Val111Ala
(Saccharomyces cerevisiae) related supports the existence of the 'Heme and NADP(H) binding
pockets'. The omission of a 20-residue PDB: 1F4J, (1QQW) segment
corresponds to the N-terminal (blue) of catalase from human
erythrocytes (HEC), or in a C-terminal
(red) domain organized with an extra flavodoxin-like
fold
topology may provide with weak coordination the N- or
C-terminal, that allows scrutiny of the origins (topology) in
this report of what would otherwise remain speculative or determined with further verification.
 The cytosine to thymidine transition of nucleotide-262 (-262C>T)
Computer analysis indicated that the two variants bound promoter the
Ile (-262 C/T) and (B) Ile-262 in
the 5'-flanking
region carrying the T allele best captured and characterized the
generation of the hydroxyl radical site in (PDB: 1DGB), (CAT) -[GLU] 330C>T transition, is known also as -262C>T. The
'T allele in comparison to the C allele' is a common C/T
polymorphism frequency in the promoter
region association was observed between genotypes for locus11p13 risk alleles acatalasemia mutation Asp
(37C>T in exon 9) was hypothetically related to the lower risk Japanese acatalasemia allele Tyr308 a single G to A (see: rs7947841 to evaluate the link to rs769214) point mutation ineducable or near exon 9 (TC,
CC, TT) of the CAT gene to which variant changes in the promoter region
C/T-262 polymorphism are more closely related to CAT T/C at codon 389 in exon 9 (rs769217)
polymorphism did not differ significantly from
those of healthy controls in both promoter (-262 C/T) and
in exonic (ASP-389 C/T)
regions of the catalase (CAT).
The cytosine to thymidine transition of nucleotide-262 (-262C>T)
Computer analysis indicated that the two variants bound promoter the
Ile (-262 C/T) and (B) Ile-262 in
the 5'-flanking
region carrying the T allele best captured and characterized the
generation of the hydroxyl radical site in (PDB: 1DGB), (CAT) -[GLU] 330C>T transition, is known also as -262C>T. The
'T allele in comparison to the C allele' is a common C/T
polymorphism frequency in the promoter
region association was observed between genotypes for locus11p13 risk alleles acatalasemia mutation Asp
(37C>T in exon 9) was hypothetically related to the lower risk Japanese acatalasemia allele Tyr308 a single G to A (see: rs7947841 to evaluate the link to rs769214) point mutation ineducable or near exon 9 (TC,
CC, TT) of the CAT gene to which variant changes in the promoter region
C/T-262 polymorphism are more closely related to CAT T/C at codon 389 in exon 9 (rs769217)
polymorphism did not differ significantly from
those of healthy controls in both promoter (-262 C/T) and
in exonic (ASP-389 C/T)
regions of the catalase (CAT).  Tyr 370
resolves the 25 A-long (hydrogen peroxide) channel a constriction or
narrowing of the channel leading to the heme cavity ('Parameters)
situated in the entrance channel to a heme protoporphyrin (ZnPPIX)
(PDB: 1H6N) from a heme group, capable of heme biosynthesis'
in a wide range of organisms convert it into into heme b,
protoporphyrin IX-heme.
Two channels lead close to the distal side. A third channel
reaching the heme proximal side
Tyr 370, Ile-262
is proposed as a the 'PDB: 1DGB - variant with a substituted residue in the ASP 178 to the (Met)
D181E variant PDB 1p80'.
These differences include the structure of the variant protein Val111Ala
(Saccharomyces cerevisiae) related supports the existence of the 'Heme and NADP(H) binding
pockets'. The omission of a 20-residue PDB: 1F4J, (1QQW) segment
corresponds to the N-terminal (blue) of catalase from human
erythrocytes (HEC), or in a C-terminal
(red) domain organized with an extra flavodoxin-like
fold
topology may provide with weak coordination the N- or
C-terminal, that allows scrutiny of the origins (topology) in
this report of what would otherwise remain speculative or determined with further verification.
Tyr 370
resolves the 25 A-long (hydrogen peroxide) channel a constriction or
narrowing of the channel leading to the heme cavity ('Parameters)
situated in the entrance channel to a heme protoporphyrin (ZnPPIX)
(PDB: 1H6N) from a heme group, capable of heme biosynthesis'
in a wide range of organisms convert it into into heme b,
protoporphyrin IX-heme.
Two channels lead close to the distal side. A third channel
reaching the heme proximal side
Tyr 370, Ile-262
is proposed as a the 'PDB: 1DGB - variant with a substituted residue in the ASP 178 to the (Met)
D181E variant PDB 1p80'.
These differences include the structure of the variant protein Val111Ala
(Saccharomyces cerevisiae) related supports the existence of the 'Heme and NADP(H) binding
pockets'. The omission of a 20-residue PDB: 1F4J, (1QQW) segment
corresponds to the N-terminal (blue) of catalase from human
erythrocytes (HEC), or in a C-terminal
(red) domain organized with an extra flavodoxin-like
fold
topology may provide with weak coordination the N- or
C-terminal, that allows scrutiny of the origins (topology) in
this report of what would otherwise remain speculative or determined with further verification.
Biological Xenobiotic Extracts Applications of note In the presence
of Catalase:
green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG)
Yamamoto T, Lewis J, Wataha J, Dickinson D, Singh B, Bollag WB, Ueta
E, OsakiT, Athar M, Schuster G, Hsu S. Roles of catalase and hydrogen
peroxide in greentea polyphenol-induced chemopreventive effects. J
Pharmacol Exp Ther. 2004Jan;308(1):317-23. Epub 2003 Oct 20. PubMed
PMID: 14569057.Furukawa A, Oikawa S, Murata M, Hiraku Y, Kawanishi S.
(-)-Epigallocatechingallate causes oxidative damage to isolated and
cellular DNA. Biochem Pharmacol.2003 Nov 1;66(9):1769-78. PubMed PMID:
14563487.*
Trigonella (Fenugreek)
Mohammad S, Taha A, Bamezai RN, Basir SF, Baquer NZ. Lower doses of
vanadatein combination with trigonella restore altered carbohydrate
metabolism andantioxidant status in alloxan-diabetic rats. Clin Chim
Acta. 2004Apr;342(1-2):105-14. Erratum in: Clin Chim Acta. 2010 Aug
5;411(15-16):1158.Mohamad, Sameer [corrected to Mohammad, Sameer].
PubMed PMID: 15026271.
Aegle marmelos
Khan TH, Sultana S. Antioxidant and hepatoprotective potential of
Aeglemarmelos Correa. against CCl4-induced oxidative stress and early
tumor events. JEnzyme Inhib Med Chem. 2009 Apr;24(2):320-7. doi:
10.1080/14756360802167754 .PubMed PMID: 18830880.
Centella asiatica
Flora SJ, Gupta R. Beneficial effects of Centella asiatica aqueous extractagainst arsenic-induced oxidative stress and essential metal status in rats.Phytother Res. 2007 Oct;21(10):980-8. PubMed PMID: 17600859.
Flora SJ, Gupta R. Beneficial effects of Centella asiatica aqueous extractagainst arsenic-induced oxidative stress and essential metal status in rats.Phytother Res. 2007 Oct;21(10):980-8. PubMed PMID: 17600859.
Daidzein
Mishra P, Kar A, Kale RK. Prevention of chemically induced mammarytumorigenesis by daidzein in pre-pubertal rats: the role of peroxidative damageand antioxidative enzymes. Mol Cell Biochem. 2009 May;325(1-2):149-57. doi:10.1007/s11010-009-0029-1. Epub 2009 Feb 13. PubMed PMID: 19214712.
Mishra P, Kar A, Kale RK. Prevention of chemically induced mammarytumorigenesis by daidzein in pre-pubertal rats: the role of peroxidative damageand antioxidative enzymes. Mol Cell Biochem. 2009 May;325(1-2):149-57. doi:10.1007/s11010-009-0029-1. Epub 2009 Feb 13. PubMed PMID: 19214712.
Capparis
Yadav P, Sarkar S, Bhatnagar D. Action of capparis decidua againstalloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997Sep;36(3):221-8. PubMed PMID: 9367667.
Yadav P, Sarkar S, Bhatnagar D. Action of capparis decidua againstalloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997Sep;36(3):221-8. PubMed PMID: 9367667.
Retinal
Kannan R, Jin M, Gamulescu MA, Hinton DR. Ceramide-induced apoptosis: role ofcatalase and hepatocyte growth factor. Free Radic Biol Med. 2004 Jul15;37(2):166-75. PubMed PMID: 15203188.
Kannan R, Jin M, Gamulescu MA, Hinton DR. Ceramide-induced apoptosis: role ofcatalase and hepatocyte growth factor. Free Radic Biol Med. 2004 Jul15;37(2):166-75. PubMed PMID: 15203188.
Retinol
Cemek M, Caksen H, Bayiroğlu F, Cemek F, Dede S. Oxidative stress andenzymic-non-enzymic antioxidant responses in children with acute pneumonia. CellBiochem Funct. 2006 May-Jun;24(3):269-73. PubMed PMID: 16634091.
Cemek M, Caksen H, Bayiroğlu F, Cemek F, Dede S. Oxidative stress andenzymic-non-enzymic antioxidant responses in children with acute pneumonia. CellBiochem Funct. 2006 May-Jun;24(3):269-73. PubMed PMID: 16634091.
Diallyl disulfide (Allicin)
Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G.Diallyl sulfide enhances antioxidants and inhibits inflammation through theactivation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. EurJ Pharmacol. 2009 Mar 15;606(1-3):162-71. doi: 10.1016/j.ejphar.2008.12.055. Epub2009 Jan 19. PubMed PMID: 19374873.
Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G.Diallyl sulfide enhances antioxidants and inhibits inflammation through theactivation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. EurJ Pharmacol. 2009 Mar 15;606(1-3):162-71. doi: 10.1016/j.ejphar.2008.12.055. Epub2009 Jan 19. PubMed PMID: 19374873.
Leucas aspera (Catechin, EGCG)
Kripa KG, Chamundeeswari D, Thanka J, Uma Maheswara Reddy C. Modulation ofinflammatory markers by the ethanolic extract of Leucas aspera in adjuvantarthritis. J Ethnopharmacol. 2011 Apr 12;134(3):1024-7. doi:10.1016/j.jep.2011.01.010. Epub 2011 Jan 18. PubMed PMID: 21251972.
Kripa KG, Chamundeeswari D, Thanka J, Uma Maheswara Reddy C. Modulation ofinflammatory markers by the ethanolic extract of Leucas aspera in adjuvantarthritis. J Ethnopharmacol. 2011 Apr 12;134(3):1024-7. doi:10.1016/j.jep.2011.01.010. Epub 2011 Jan 18. PubMed PMID: 21251972.
Urtica dioica (nettle suppliment)Ozen T, Korkmaz H. Modulatory effect of Urtica dioica L. (Urticaceae) leaf
extract on biotransformation enzyme systems, antioxidant enzymes, lactatedehydrogenase and lipid peroxidation in mice. Phytomedicine. 2003;10(5):405-15.PubMed PMID: 12834006.
extract on biotransformation enzyme systems, antioxidant enzymes, lactatedehydrogenase and lipid peroxidation in mice. Phytomedicine. 2003;10(5):405-15.PubMed PMID: 12834006.
Justicia adhatoda
Singh RP, Padmavathi B, Rao AR. Modulatory influence of Adhatoda vesica(Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism,antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000Oct;213(1-2):99-109. PubMed PMID: 11129964.
Singh RP, Padmavathi B, Rao AR. Modulatory influence of Adhatoda vesica(Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism,antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000Oct;213(1-2):99-109. PubMed PMID: 11129964.
Phyllanthus niruri L. (Euphorbiaceae) (P. niruri)
Bhattacharjee R, Sil PC. Protein isolate from the herb, Phyllanthus niruri L.(Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride inducedliver damage via its antioxidant properties. Food Chem Toxicol. 2007May;45(5):817-26. Epub 2006 Nov 11. PubMed PMID: 17175085.
Bhattacharjee R, Sil PC. Protein isolate from the herb, Phyllanthus niruri L.(Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride inducedliver damage via its antioxidant properties. Food Chem Toxicol. 2007May;45(5):817-26. Epub 2006 Nov 11. PubMed PMID: 17175085.
Tinospora cordifolia
Sharma V, Pandey D. Protective Role of Tinospora cordifolia againstLead-induced Hepatotoxicity. Toxicol Int. 2010 Jan;17(1):12-7. doi:10.4103/0971-6580.68343. PubMed PMID: 21042467; PubMed Central PMCID: PMC2964743.
Sharma V, Pandey D. Protective Role of Tinospora cordifolia againstLead-induced Hepatotoxicity. Toxicol Int. 2010 Jan;17(1):12-7. doi:10.4103/0971-6580.68343. PubMed PMID: 21042467; PubMed Central PMCID: PMC2964743.
Aher V, Kumar Wahi A. Biotechnological Approach to Evaluate
theImmunomodulatory Activity of Ethanolic Extract of Tinospora
cordifolia Stem(Mango Plant Climber). Iran J Pharm Res. 2012
Summer;11(3):863-72. PubMed PMID:24250513; PubMed Central PMCID:
PMC3813135.
coenzyme Q10
Lee BJ, Lin YC, Huang YC, Ko YW, Hsia S, Lin PT. The relationship betweencoenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronaryartery disease. ScientificWorldJournal. 2012;2012:792756. doi:10.1100/2012/792756. Epub 2012 May 3. PubMed PMID: 22645453; PubMed CentralPMCID: PMC3356738.
Lee BJ, Lin YC, Huang YC, Ko YW, Hsia S, Lin PT. The relationship betweencoenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronaryartery disease. ScientificWorldJournal. 2012;2012:792756. doi:10.1100/2012/792756. Epub 2012 May 3. PubMed PMID: 22645453; PubMed CentralPMCID: PMC3356738.
Dietary carotenoid-rich pequi oil
Miranda-Vilela AL, Akimoto AK, Alves PC, Pereira LC, Gonçalves CA,Klautau-Guimarães MN, Grisolia CK. Dietary carotenoid-rich pequi oil reducesplasma lipid peroxidation and DNA damage in runners and evidence for anassociation with MnSOD genetic variant -Val9Ala. Genet Mol Res. 2009 Dec15;8(4):1481-95. doi: 10.4238/vol8-4gmr684. PubMed PMID: 20082261.
Miranda-Vilela AL, Akimoto AK, Alves PC, Pereira LC, Gonçalves CA,Klautau-Guimarães MN, Grisolia CK. Dietary carotenoid-rich pequi oil reducesplasma lipid peroxidation and DNA damage in runners and evidence for anassociation with MnSOD genetic variant -Val9Ala. Genet Mol Res. 2009 Dec15;8(4):1481-95. doi: 10.4238/vol8-4gmr684. PubMed PMID: 20082261.
Tinospora
cordifolia (Mango Plant Climber) extract from Tinospora
known as Tinofend Aher V, Kumar Wahi A. Biotechnological Approach to
Evaluate theImmunomodulatory Activity of Ethanolic Extract of Tinospora
cordifolia Stem(Mango Plant Climber). Iran J Pharm Res. 2012
Summer;11(3):863-72. PubMed PMID:24250513; PubMed Central PMCID:
PMC3813135.
mulberry leaf polysaccharide (MLPII)
Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao H, Huang L, Mu Z. A polysaccharideextract of mulberry leaf ameliorates hepatic glucose metabolism and insulinsignaling in rats with type 2 diabetes induced by high fat-diet andstreptozotocin. Int J Biol Macromol. 2014 Oct 11. pii: S0141-8130(14)00674-6.doi: 10.1016/j.ijbiomac.2014.09.060. [Epub ahead of print] PubMed PMID: 25316427.
Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao H, Huang L, Mu Z. A polysaccharideextract of mulberry leaf ameliorates hepatic glucose metabolism and insulinsignaling in rats with type 2 diabetes induced by high fat-diet andstreptozotocin. Int J Biol Macromol. 2014 Oct 11. pii: S0141-8130(14)00674-6.doi: 10.1016/j.ijbiomac.2014.09.060. [Epub ahead of print] PubMed PMID: 25316427.
five widely studied medicinal plants (Protandim)
Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of humansuperoxide dismutase and catalase in vivo: a fundamentally new approach toantioxidant therapy. Free Radic Biol Med. 2006 Jan 15;40(2):341-7. PubMed PMID:16413416.
Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of humansuperoxide dismutase and catalase in vivo: a fundamentally new approach toantioxidant therapy. Free Radic Biol Med. 2006 Jan 15;40(2):341-7. PubMed PMID:16413416.
melatonin
Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ. Oxidative damage tocatalase induced by peroxyl radicals: functional protection by melatonin andother antioxidants. Free Radic Res. 2003 May;37(5):543-53. PubMed PMID: 12797476.
Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ. Oxidative damage tocatalase induced by peroxyl radicals: functional protection by melatonin andother antioxidants. Free Radic Res. 2003 May;37(5):543-53. PubMed PMID: 12797476.
Protective effect of harmaline
Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalolagainst dopamine- and 6-hydroxydopamine-induced oxidative damage of brainmitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci.2001 May;13(10):1861-72. PubMed PMID: 11403679.
Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalolagainst dopamine- and 6-hydroxydopamine-induced oxidative damage of brainmitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci.2001 May;13(10):1861-72. PubMed PMID: 11403679.
horseradish peroxidase (HRP)
Shen L, Hu N. Heme protein films with polyamidoamine dendrimer: directelectrochemistry and electrocatalysis. Biochim Biophys Acta. 2004 Jan30;1608(1):23-33. PubMed PMID: 14741582.
Shen L, Hu N. Heme protein films with polyamidoamine dendrimer: directelectrochemistry and electrocatalysis. Biochim Biophys Acta. 2004 Jan30;1608(1):23-33. PubMed PMID: 14741582.
Selegiline (--)Deprenyl
Kitani K, Minami C, Isobe K, Maehara K, Kanai S, Ivy GO, Carrillo MC. Why(--)deprenyl prolongs survivals of experimental animals: increase of anti-oxidantenzymes in brain and other body tissues as well as mobilization of varioushumoral factors may lead to systemic anti-aging effects. Mech Ageing Dev. 2002Apr 30;123(8):1087-100. Review. PubMed PMID: 12044958.
Kitani K, Minami C, Isobe K, Maehara K, Kanai S, Ivy GO, Carrillo MC. Why(--)deprenyl prolongs survivals of experimental animals: increase of anti-oxidantenzymes in brain and other body tissues as well as mobilization of varioushumoral factors may lead to systemic anti-aging effects. Mech Ageing Dev. 2002Apr 30;123(8):1087-100. Review. PubMed PMID: 12044958.
Rhodiola rosea
Bayliak MM, Lushchak VI. The golden root, Rhodiola rosea, prolongs lifespanbut decreases oxidative stress resistance in yeast Saccharomyces cerevisiae.Phytomedicine. 2011 Nov 15;18(14):1262-8. doi: 10.1016/j.phymed.2011.06.010. Epub2011 Jul 30. PubMed PMID: 21802922.
Bayliak MM, Lushchak VI. The golden root, Rhodiola rosea, prolongs lifespanbut decreases oxidative stress resistance in yeast Saccharomyces cerevisiae.Phytomedicine. 2011 Nov 15;18(14):1262-8. doi: 10.1016/j.phymed.2011.06.010. Epub2011 Jul 30. PubMed PMID: 21802922.
Carnitine
Kiziltunc A, Coğalgil S, Cerrahoğlu L. Carnitine and antioxidants levels inpatients with rheumatoid arthritis. Scand J Rheumatol. 1998;27(6):441-5. PubMedPMID: 9855215.
Kiziltunc A, Coğalgil S, Cerrahoğlu L. Carnitine and antioxidants levels inpatients with rheumatoid arthritis. Scand J Rheumatol. 1998;27(6):441-5. PubMedPMID: 9855215.
Syzygium cumini
De Bona KS, Bellé LP, Sari MH, Thomé G, Schetinger MR, Morsch VM, Boligon A,
Athayde ML, Pigatto AS, Moretto MB. Syzygium cumini extract decrease adenosine
deaminase, 5'nucleotidase activities and oxidative damage in platelets of
diabetic patients. Cell Physiol Biochem. 2010;26(4-5):729-38. doi:
10.1159/000322340. Epub 2010 Oct 29. PubMed PMID: 21063110.
Athayde ML, Pigatto AS, Moretto MB. Syzygium cumini extract decrease adenosine
deaminase, 5'nucleotidase activities and oxidative damage in platelets of
diabetic patients. Cell Physiol Biochem. 2010;26(4-5):729-38. doi:
10.1159/000322340. Epub 2010 Oct 29. PubMed PMID: 21063110.




![The Hidden Hand ‘characterized the essential identity arXiv readership G[oogle] S[cholar]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjF1lIpjyufTCDmFwS323y5r25_SeU2xKmuE_Bd_imffJItOx_NgeQEPFZVISAz5S8VmG_tAe2zvciFekArBNVHpATGN_hqYl0ax0_atcI2PZeHTq2C-QTwhZrTr3F5fTuxG7w1gQ/s400/work+by+Dai+Dudu,+Li+Tiezi,+and+Zhang+An+in+2006.jpg) [N]eo[C]on
[N]eo[C]on


